In modern times, the world has not experienced anything quite like the COVID-19 pandemic, which spread across the globe with alarming ferocity. As of January 11, 2021, there have been more than 91 million reported cases and nearly 2 million deaths according to Worldometer. Also unprecedented has been the rapid development of effective vaccines and sophisticated cold chains thanks to the effort of governments, leading pharmaceutical companies, logistics companies, and others. There are still details to consider in this complex rollout. And that includes the optimal pallet solution.
Last December, a month where many anticipate the arrival of the holiday season, there was another cause for celebration among our pandemic-weary nation and a world. Not one, but two COVID-19 virus vaccines were ready for distribution, one from Pfizer and its partner, BioNTech, and the other from Moderna. Both showed essentially equal degrees of effectiveness: 95% efficacy for the Pfizer vaccine at preventing symptomatic COVID-19 infection, 94.1% for the Moderna vaccine. Both required two shots, a priming dose, and a booster shot.

There is one more commonality. Both vaccines share a challenging cold chain—the term used to describe the conditions under which vaccines must be stored from the point of manufacture through various points of distribution and the endpoint for delivery to patients—with one difference. The Pfizer vaccine’s handling requirements are extreme. Whereas the Moderna vaccine must be shipped and stored at -4 Fahrenheit, Pfizer’s required cold chain temperature is -94 Fahrenheit.
As a simple point of reference, Moderna’s cold chain temperature is comparable to that needed to keep a pint of Haagen-Daz frozen, something any home refrigerator freezer can manage. The Pfizer vaccine requires special ultracold freezers. At -94 degrees Fahrenheit, that’s 20 degrees colder than the highest peak of Antarctica (-74 degrees).
According to an article in Science News, most vaccines don’t require freezing at all, but both Pfizer and Moderna’s vaccines are a new type of biological vaccine based on messenger RNA (mRNA) whose role it is to communicate with human cells. In this case, mRNA tells the immune system to attack the coronavirus should it appear in an individual’s body. mRNA-based vaccines are extremely fragile and the ultra-frosty temperatures keep them from breaking down and becoming useless. Thus, consistent application of this deep freeze ensures freshness and effectiveness.
Getting millions of vials of vaccines to hospitals, clinics, and pharmacies across the United States and around the world will not be easy. Anticipating concerns, Pfizer instructed health officials early on how the vaccine can be stored: in special shipping containers that can be recharged with dry ice for 15 days and stay refrigerated for another five days after thawing.
Using the United State as just one example of the global vaccine roll-out, the federal government has recently expanded efforts to make COVID-19 vaccines available to the public as rapidly as possible. An initial goal is to produce and deliver 300 million doses of vaccines in January alone as part of a broader strategy to accelerate the development, manufacturing and distribution.
Working closely with the Centers for Disease Control (CDC) and other logistics and supply chain partners, the federal government has in place a centralized system to order, distribute, and track COVID-19 vaccines. The system continues to be adjusted and optimized to avoid bottlenecks and facilitate the “last mile” of distribution.
Currently, vaccine providers receive vaccines from CDC’s centralized distributor or directly from a vaccine manufacturer following a highly orchestrated plan that includes:
- Logistics plans developed and tested with manufacturers and commercial partners;
- How best to receive, store, and handle vaccines at very specific temperatures; and
- Reporting on vaccine inventory, administration, and safety using a variety of new and enhanced data systems.

As mentioned earlier, depending on the capacity required, the COVID-19 vaccine cold chain requires specialized cold or freezer rooms, freezers, refrigerators, cold boxes, and, in some cases, refrigerated trucks and planes for transportation. At the same time, it’s essential to monitor and record the temperature of vaccines across the supply chain.
The complexities are immense. In a New York Times article dating back to September 2020, J. Stephen Morrison, senior vice president at the Center for Strategic and International Studies, said,“ There’s no getting around it. These (vaccines) have stark temperature demands that will constrain access and delivery.”
The New York Times article goes on to state that “much of the work (actual distribution) will fall to companies outside the medical and drug industries. The major U.S. logistics companies, including UPS and FedEx, already have networks of freezers that they use to ship perishable food and medical supplies. The companies have experience shipping vaccines for other illnesses, including the seasonal flu.”

However, neither UPS nor FedEx has seen anything like the scope of the COVID-19 vaccine rollout, including the ultra-low temperature (ULT) cold chain requirements. UPS constructed a freezer farm in Louisville, Ky., the company’s largest hub, where it can store millions of doses at subzero temperatures. The freezer farm is filled with rows of industrial ULT freezers, each capable of holding 48,000 vials. FedEx also added freezers that can maintain temperatures as low as -80 degrees Celsius at its hubs in Memphis, Indianapolis, and Paris. Included in Fedex’s efforts are refrigerated trailers that can be used for vaccines that need to be chilled rather than frozen.
To package the vaccines themselves, Pfizer has developed a special container that is roughly the size of a large cooler that hold a couple hundred glass vials, each containing 10 to 20 doses of vaccine. The boxes are equipped with GPS-enabled thermal sensors, allowing Pfizer to know where the boxes are and how cold they are. It is a mammoth effort with an incredible number of details.
So where do pallets fit into this complex rollout given the need to ensure vaccine integrity and hygiene, the new requirement of ultra-low temperatures, and the traceability of pallets and their precious cargo? In no other industry is the demand for sanitation and hygiene higher than it is in pharmaceuticals. Companies making everything from over-the-counter medication and now the COVID19 vaccines are expected by the public to manufacture and deliver products that are safe and effective. Therefore, even something as seemingly simple as a pallet must be scrutinized. There are basically three choices:
Wood Pallets
Although a supply chain stalwart, wood pallets pose the most challenges to the safe conveyance of pharmaceutical products like the COVID-19 vaccine. The porous nature of wood presents the greatest risk to product integrity as water and other liquids absorbed into a wooden pallet can become a breeding ground for bacteria and microorganisms. Cleaning between uses doesn’t help matters. It’s just another round of moisture that compromises the wood.
The cause for concern is real. Following the 2010 recall of E. coli-tainted romaine lettuce, the National Consumers League conducted tests on pallets to see if they could be potential carriers of harmful bacteria.

Ten percent of the pallets tested were found to have E. coli present, while 2.9% tested positive for listeria, a highly aggressive foodborne pathogen.
Another obvious disqualifier is that wood pallets are prone to breakage, whether from the weight of the load or repeated handling. Wood particles, entire boards, and loose nails can cause contamination and damage to freezers and another protective packaging. These loose fragments also cause contamination risks to pharmaceutical manufacturing facilities, distribution centers, and refrigerated trailers.
Finally, the ultra-low temperatures needed to protect the effectiveness of COVID-19 vaccines likely disqualify wood pallets. Extreme shifts in temperature cause wood to warp. The more out of spec the wood pallets become, the higher the risk of breakage during handling. So definitely not the ideal choice.
Plastic Pallets
On the surface, plastic pallets would seem like a good option for the pharmaceutical industry. Plastic pallets are made primarily of two different resins, polyethylene and polypropylene. Both materials are inert so very little sticks to it. This low coefficient of friction can make plastic pallets slippery, which presents a potential hazard when handling or when stacked. Unlike wood, plastic pallets are easily washed and disinfected, which supports a clean manufacturing environment. They are durable, with high impact and tensile strength that supports pallet longevity.
This resilience is certainly attractive when considering the millions of vaccine doses making their way through the COVID-19 cold chain. But here’s the drawback and it’s a big one. When used in ultra-low temperature environments, plastic pallets can become harder, stiffer, and more brittle and thus more prone the breakage. In particular, polypropylene pallets are inherently more brittle and thus unsuitable for use in freezing temperatures. Given the speed and efficiency with which the federal government, pharmaceutical companies, and healthcare providers are demanding to accelerate access to the COVID-19 vaccine, plastic pallets may pose avoidable risks.

Composite Pallets
The third contender for consideration are composite pallets, a category defined by RM2’s smart pallets, marketed under the name BLOCKpal, that have been specifically engineered for the most demanding supply chains. The COVID-19 vaccines, those manufactured by Pfizer and Moderna certainly fall into that category.
RM2’s composite pallets are composed of resins reinforced with fiberglass, resulting in a nonporous material naturally resistant to bacteria, chemicals and pests. The composite material is non-shedding and therefore doesn’t produce dust or debris, minimizing risk of pallet-related foreign material that would compromise hygienic environments. The composite BLOCKpal pallets can be cleaned and disinfected to ISO 22000 standards and reused upwards of 150 times. Each time a BLOCKpal pallet is cleaned, RM2 records the RLU readings and makes them available to customers for audit trail.
Unlike plastic pallets, these composite pallets embrace ultra-low temperatures demanded by biologic vaccines without becoming brittle. In fact, the BLOCKpal pallets’ tolerance range of – 40 degrees to 170 degrees Fahrenheit exceeds the range of other pallets materials by more than 20 degrees each way.
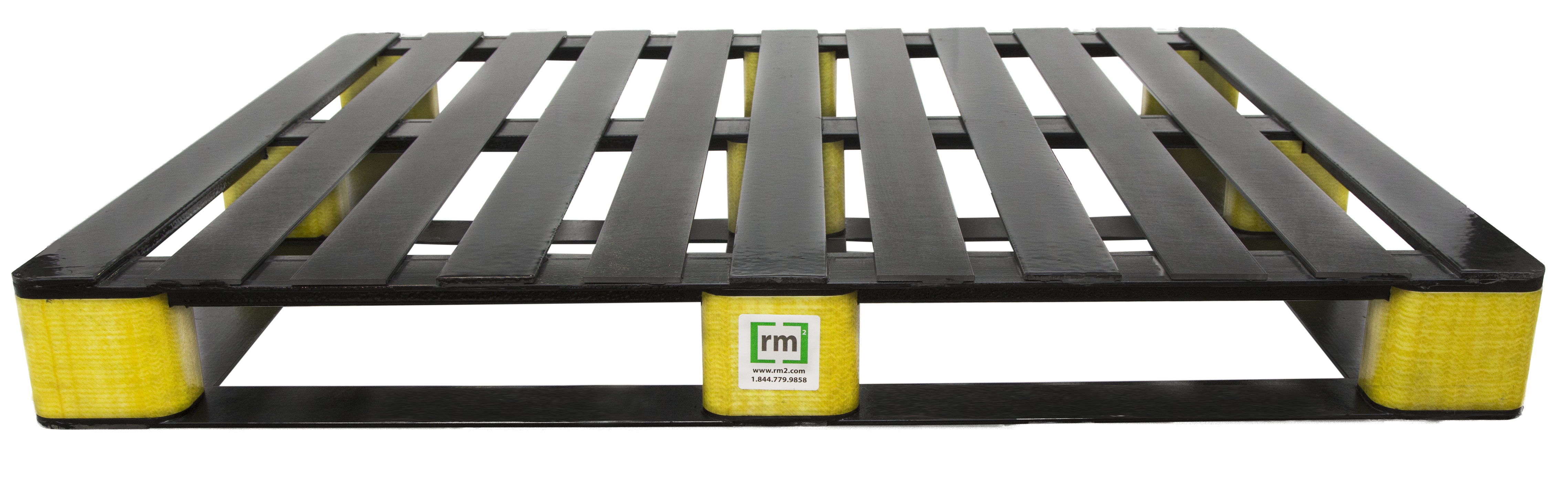
Finally, RM2’s composite pallets bring something typical wood and plastic pallets don’t: each pallet includes embedded RM2 ELIoT® IoT sensor technology that enables automated tracking in real time of chain of custody, temperature, location, and shock events for supply chain visibility critical to the secure, rapid, on-time delivery of COVID-19 vaccines.
Putting Global Health Back on Track
The last 12 months have been epic, both in the threat to public health caused by COVID-19 and the breath-taking speed in which countries have responded with effective, life-saving vaccines and therapeutics. There is much to be proud of and much work to be done bringing these vaccines with their full-force protected, to the world. No detail is too small. This is why it is so important to select the right pallets, composite pallets for the extreme performance required by the COVID-19 vaccines’ cold chain.
